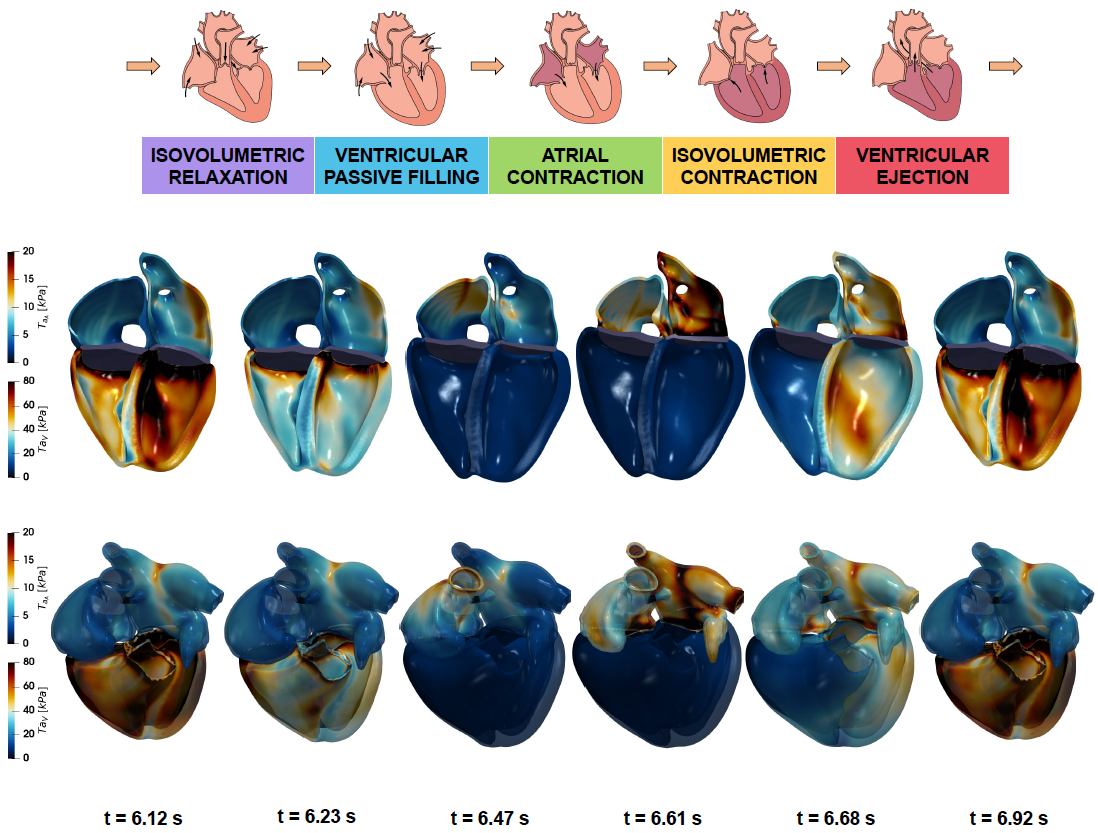
A new MOX report entitled “A comprehensive and biophysically detailed computational model of the whole human heart electromechanics” by Fedele, M.; Piersanti, R.; Regazzoni, F.; Salvador, M.; Africa, P. C.; Bucelli, M.; Zingaro, A.; Dede’, L.; Quarteroni, A. has appeared in the MOX Report Collection.
The report can be donwloaded at the following link:
https://www.mate.polimi.it/biblioteca/add/qmox/52/2022.pdf
Abstract: While ventricular electromechanics is extensively studied in both physiological and pathological conditions, four-chamber heart models have only been addressed recently; most of these works however neglect atrial contraction. Indeed, as atria are characterized by a complex anatomy and a physiology that is strongly influenced by the ventricular function, developing computational models able to capture the physiological atrial function and atrioventricular interaction is very challenging. In this paper, we propose a biophysically detailed electromechanical model of the whole human heart that considers both atrial and ventricular contraction. Our model includes: i) an anatomically accurate whole-heart geometry; ii) a comprehensive myocardial fiber architecture; iii) a biophysically detailed microscale model for the active force generation; iv) a 0D closed-loop model of the circulatory system, fully-coupled with the mechanical model of the heart; v! ) the fun damental interactions among the different \textit{core models}, such as the mechano-electric feedback or the fibers-stretch and fibers-stretch-rate feedbacks; vi) specific constitutive laws and model parameters for each cardiac region. Concerning the numerical discretization, we propose an efficient segregated-intergrid-staggered scheme and we employ recently developed stabilization techniques – regarding the circulation and the fibers-stretch-rate feedback – that are crucial to obtain a stable formulation in a four-chamber scenario. We are able to reproduce the healthy cardiac function for all the heart chambers, in terms of pressure-volume loops, time evolution of pressures, volumes and fluxes, and three-dimensional cardiac deformation, with unprecedented matching (to the best of our knowledge) with the expected physiology. We also show the importance of considering atrial contraction, fibers-stretch-rate feedback and suitable stabilization techniques, by comparing the re! sults obt ained with and without these features in the model. The proposed model represents the state-of-the-art electromechanical model of the iHEART ERC project – an Integrated Heart Model for the Simulation of the Cardiac Function – and is a fundamental step toward the building of physics-based digital twins of the human heart.